The need for accurate, objective and reproducible measurements of body composition has long been of interest in livestock production. Real-time Ultrasound (RTU) scanning is not only a measure of the physiological makeup of an animal, but also an indication of carcass traits (Ribeiro, et al., 2014) which are economically important in beef production. RTU scanning is a non-invasive and portable technology used to measure body composition in live animals. RTU scanning devices can be used to record the eye-muscle area (EMA), intramuscular fat (IMF) and rib- and rump fat depth in live animals (Figure1) as opposed to waiting the entire lifespan of the animal to record carcass traits at slaughter. The value of recording RTU scanning data is emphasised by the genetic parameters, contribution to Estimated Breeding Value (EBV) calculations and the importance of fat deposition in beef production.
Figure 1: Diagram of approximate anatomical locations for ultrasound measurements obtained from Knight (2018) – Ultrasound measurements are collected at three points on the animal (Silcox, 2002).
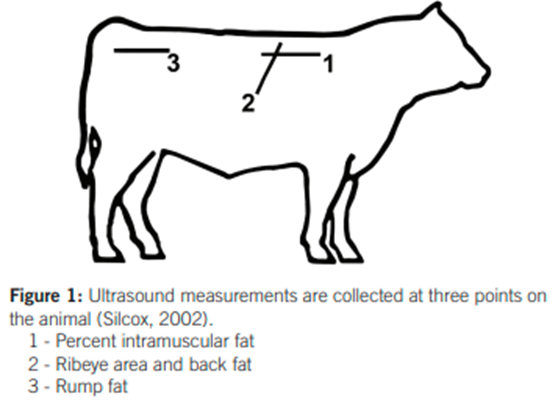
RTU scanning can be performed on live animals whereby various anatomical locations are scanned using ultrasound to quantitatively measure body composition (Table 1). The heritability of traits recorded using RTU scanning is moderate to high (Seroba, et al., 2011). Furthermore, there is a strong correlation between carcass data and RTU measurements for body composition (Ribeiro, et al., 2014). This suggests that RTU scanning data may be a good indicator of carcass traits (Ribeiro, et al., 2014) and that genetic progress in ultrasound indicators of carcass traits may be possible (Seroba, et al., 2011) through selection.
In order to calculate EBVs for traits recorded using RTU scanning and thereby aid the breeder in selecting animals based on the desired estimated genetic potential for each respective trait, it is paramount that this data be recorded. It should be noted that the optimal level of each EBV and combinations thereof depends on the specific breeding objective and the correlations between traits should always be considered for a balanced approach in a specific breeding objective. For more information on BREEDPLAN Carcass EBVs and scan data, visit the BREEDPLAN Help Centre (https://breedplan.une.edu.au/help-centre/). Table 1 provides a summary of traits recorded using RTU scanning and an explanation of what each trait may represent.
Table 1: A summary of traits recorded using Real-time Ultrasound scanning and an explanation of what each trait may represent.
| Trait | Anatomical location | Unit of measurement | Explanation of what each trait may represent |
| Eye-muscle area (EMA) | Cross-sectional area of longissimus dorsi muscle between the 12th and 13th rib | Square centimeters (cm2) | Overall muscling |
| Intramuscular fat (IMF) | Longissimus dorsi muscle between the 12th and 13th rib | Percentage (%) | Meat quality (marbling) |
| Rib-fat depth | Subcutaneous fat depth of the longissimus dorsi muscle at the 12/13th rib site | Millimeters (mm) | Fat coverage |
| Rump-fat depth | Subcutaneous fat depth at the P8 rump site | Millimeters (mm) | Fat coverage |
In addition, the distribution of fat and muscle tissue (body composition) in animals provides useful information in terms of animal growth. Maturity and physiological age are closely related to the development of bone, muscle and fat tissue in relation to each other as depicted by the animal growth curve (Graph 1). In simple terms bone and muscle tissue are deposited at a higher rate compared to fat tissue in early growth. Whereas in later growth and around maturity, fat tissue is deposited at a higher rate (Graph 1). While fat deposition does occur throughout growth, it begins with kidney fat and ends with intramuscular fat deposition.
Graph 1: Growth curve obtained from Hossner (2005) – Growth rates of body regions and tissues during development. Adapted from Hammond (1955).
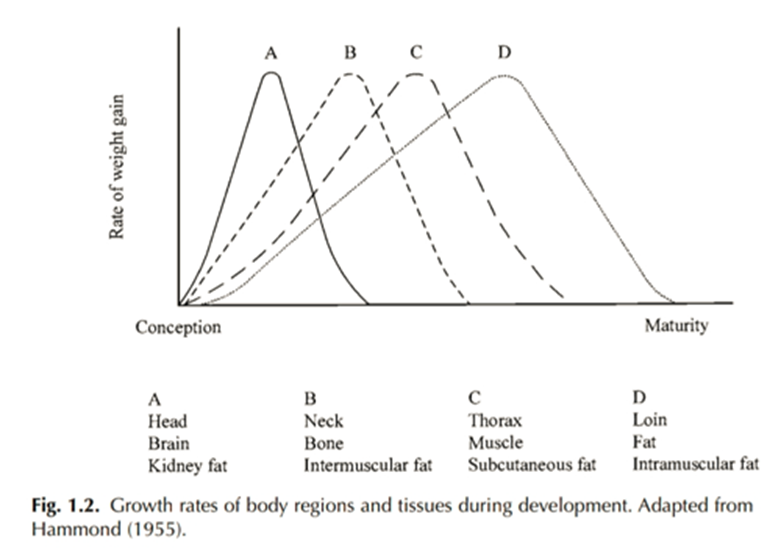
The animal growth curve tends to follow the same pattern as seen in Graph 1. However, the chronological age for each point of growth is said to differ depending on maturity type. Moloney & McGee (2017) state that in animals slaughtered at the same chronological age, later maturing animals are leaner compared to earlier maturing animals. This is because later maturing animals deposit fat later (chronologically) than earlier maturing animals. Fat deposition plays an important role in carcass quality, and this is of particular interest in extensive production systems where animals are fattened over a longer period compared to intensive production systems. RTU scanning data of body composition may therefore be a useful tool in determining fat deposition, the subsequent development of carcass characteristics and slaughter end point.
According to the Livestock Registering Federation (LRF) Test Plan, RTU scanning must be performed by an accredited technician when animals are between 300-800 days of age. It is advised that all animals being scanned are in good condition to detect the variation in measurements between animals. Both bulls and heifers can be scanned, provided there is a minimum of 10 animals per farm and that all animals being scanned are from the same contemporary group.
To utilise the LRF Scanning Services, the breeder or test station must book with the LRF office by completing a booking form. The booking form and protocol are available on the LRF website (http://www.lrf.co.za/lrf-ts/#rtu-scanning-services) which must be completed and submitted to the LRF office. RTU scanning bookings for the second half of the year are currently open. For more information and bookings regarding the LRF Scanning Services, please contact Izaan du Plooy via office@lrf.co.za or +27 81 844 4853.
Bibliography
- Botes, F., 2021. Real-time ultrasound scanning, s.l.: Stockfarm.
- de Vos, J., 2018. A Genome wide association study of carcass traits based on Real Time Ultrasound in South African Nguni cattle (Dissertation). University of Pretoria.
- Hossner, K. L., 2005. Development of Muscle, Skeletal System and Adipose Tissue. In: Hormonal regulation of farm animal growth. Oxfordshire: CABI Publishing, pp. 55-93.
- Knight, C. H., 2018. Using Live Animal Carcass Ultrasound in Beef Cattle, s.l.: University of Georgia in cooperation with Fort Valley State University, the U.S. Department of Agriculture, and counties of the state.
- Moloney, A. P. & McGee, M., 2017. Factors Influencing the Growth of Meat Animals. In: F. Toldra, ed. Lawrie´s Meat Science. s.l.:Elsevier Science & Technology, pp. 19-47.
- Ribeiro, F. R. B. et al., 2014. Comparison of real-time ultrasound measurements for body composition traits to carcass and camera data in feedlot steers. The Professional Animal Scientist, Volume 30, pp. 587-601.
- Seroba, M. M., Maiwashe, A., Nephawe, K. A. & Norris, D., 2011. Genetic parameter estimates for live animal ultrasound measures of carcass traits in South African Angus cattle. South African Journal of Animal Science, 41(3), pp. 243-249.

